
Colic
Extensive clinical studies have proven Bio- Gaia Drops containing Limosilactobacillus reuteri Protectis /TM to be totally safe for babies, from the very first day of life.
The information on this part of the website is intended only for health care professionals. I am an expert within the meaning of Act No. country xy. I herewith confirm that I am a professional according to this definition.
Unfortunately, as you are not a health care professional we cannot provide you this information.
The first such study, published in the medical journal Pediatrics (2007 ), showed that after 28 days of treatment with BioGaia Drops average crying time for colicky babies had decreased from 159 minutes to only 51 minutes, and that fully 95% of babies in the study showed marked improvements in crying time. In 2012 another study also published in the journal of Pediatrics showed a decrease in crying time of 56%! BioGaia Drops contributed not only to babies' comfort and happiness, but also to the harmony of the entire family.
A just released study of 468 healthy newborns shows that infants given the probiotic BioGaia (Limosilactobacillus reuteri Protectis /TM) cried less than half as long as infants given a placebo. The infants given BioGaia drops also had significantly fewer daily regurgitations and were less constipated compared to infants in the placebo group. As a result, the use of BioGaia drops proved to be cost saving for both parents and the community. Get an overview of all studies with BioGaia (Limosilactobacillus reuteri Protectis) proven effective for colicky babies.
Containing Limosilactobacillus reuteri Protectis, BioGaia Pobiotic Drops promote the growth of good bacteria in the infant’s intestinal tract, re-establishing a healthy bacterial balance and thus significantly reduce the average daily crying time and even show a prophylactic effect in avoiding colic, constipation and regurgitation.
drops daily for at least 4 weeks – or ideally from the first day of life!
Savino, Pelle, Oggero. J Pediatr 2007;119:e124-e130
The intake of BioGaia (L.reuteri) resulted in significantly less crying time within one week of treatment, compared to standard therapy in these infants. This effect was even more pronounced at the end of the 4-weeks study.
No side-effects were observed in either group.
Prospective, randomized, double blind, study against Simethicon
90 breastfed colicky infants aged 11-80 days
Five drops per day of BioGaia (Limosilactobacillus reuteri )(n=40) or simethicone (n=40) 60 mg/day for 21 days
Szajewska, Gyrczuk, Horvath. J Pediatr 2013;162:257-262.
Significant improved family quality of life with BioGaia.

All outcome measures significantly improved throughout intervention and maintained during follow-up.
Highly significant improvements in parents/ family quality of life with L. reuteri Protectis.
The proof of the effectiveness of L. reuteri Protectis in colic is again confirmed and now very strong.
Indrio et al. JAMA Pediatr. JAMA Pediatr.
Published online January 13, 2014. doi:10.1001/jamapediatrics.2013.4367
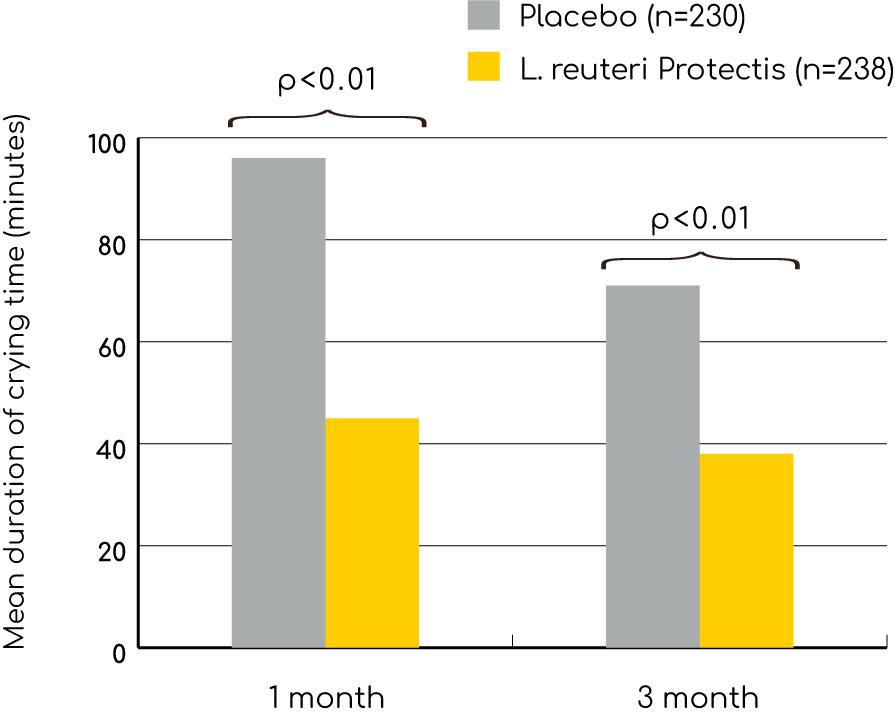
The study was a placebo-controlled, double blind, multicentre trial performed in nine neonatology units in Italy. 589 patients were randomly allocated to receive 1x108cfu of the probiotic Limosilactobacillus reuteri Protectis (BioGaia drops) or placebo daily for 90 days. Of these 468 infants completed study, 238 in the Limosilactobacillus reuteri Protectis group and 230 in the placebo group. All included infants were healthy at study start.