
Traveller’s diarrhoea
Clinical studies show strong evidence that children and adults with acute gastroenteritis caused by various causative agents while traveling benefit from the administration of BioGaia ProTectis.
The information on this part of the website is intended only for health care professionals. I am an expert within the meaning of Act No. country xy. I herewith confirm that I am a professional according to this definition.
Unfortunately, as you are not a health care professional we cannot provide you this information.
It is defined as three or more unformed stools in 24 hours passed by a traveler, commonly accompanied by abdominal cramps, nausea, and bloating. The primary source of infection is ingestion of fecally contaminated food or water.
Several systematic reviews have found probiotics to be effective in reducing the duration of diarrhoea in children and adults.
Amongst those Shornikova showed that after 2days of treatment with BioGaia 74 % of the patients have been free from watery diarrhoea and vomiting - published 1997 in the medical journal J. Pediatr. Gastroenterol. Nutr. Also the study from Francavilla published in 2012 showed the efficacy and safety of BioGaia Protectis while treating children with acute watery diarrhoea.
Containing Limosilactobacillus reuteri Protectis, BioGaia Pobiotic Drops and Tablets promote the growth of good bacteria in the intestinal tract, re-establishing a healthy bacterial balance and thus significantly reduce the duration of watery diarrhoea and even increase the resistance.
Shornikova AV et al. J. Pediatr. Gastroenterol. Nutr. 1997;24:399-404.
Significant faster recovery from watery diarrhoea in hospitalised children supplemented with Limosilactobacillus reuteri Protectis.
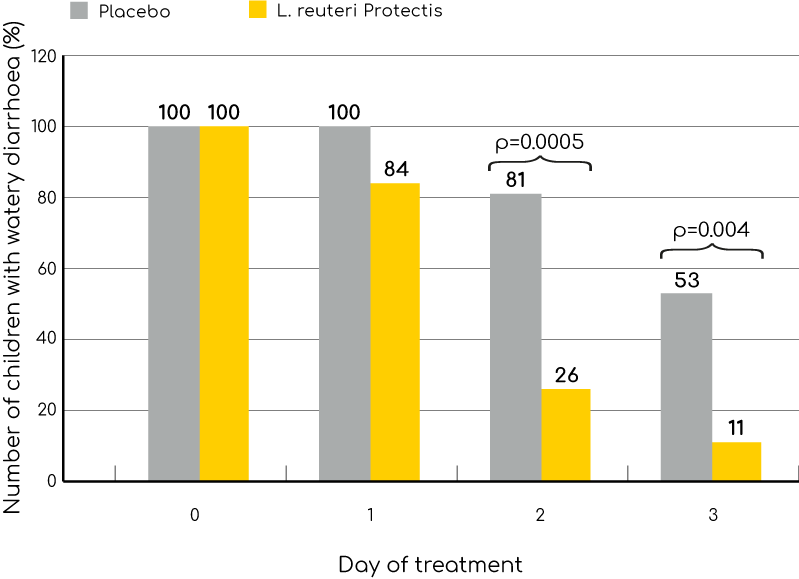
The benefits of L. reuteri (BioGaia) were observed within 24 hours of onset of treatment.
A significant effect was apparent from the second day of treatment when 74% of patients receiving BioGaia were free from watery diarrhoea compared to 19% in the control group.
Vomiting practically stopped after the first day of treatment after the intake of L. reuteri (Bio- Gaia) , while it continued in the placebo group.
Prospective, randomized, placebo controlled study
40 children aged 6-36 months hospitalised due to acute diarrhoea , 75% affected by Rotavirus
1010-1011 CFU Limosilactobacillus reuteri (BioGaia) (n=19) or placebo (n=21) for 5 days
Both received standard rehydration therapy.
Francavilla R et al. (2012) Randomised clinical trial: Aliment Pharmacol Ther. 36: 363-369.

reuteri Protectis (BioGaia) significantly reduced the duration of watery diarrhoea as compared with placebo
Children receiving L. reuteri Protectis (BioGaia) had significantly lower relapse rate of diarrhoea.