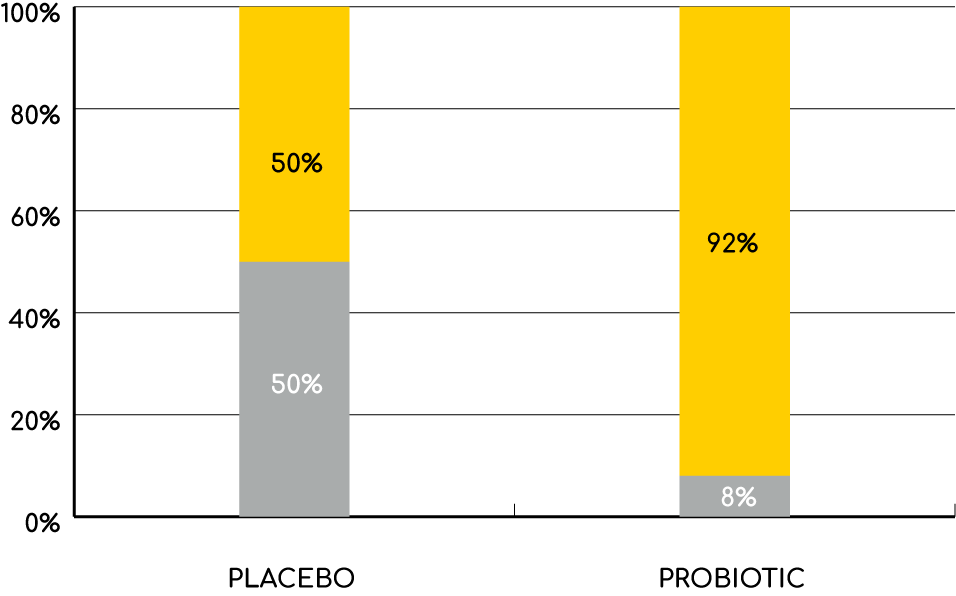
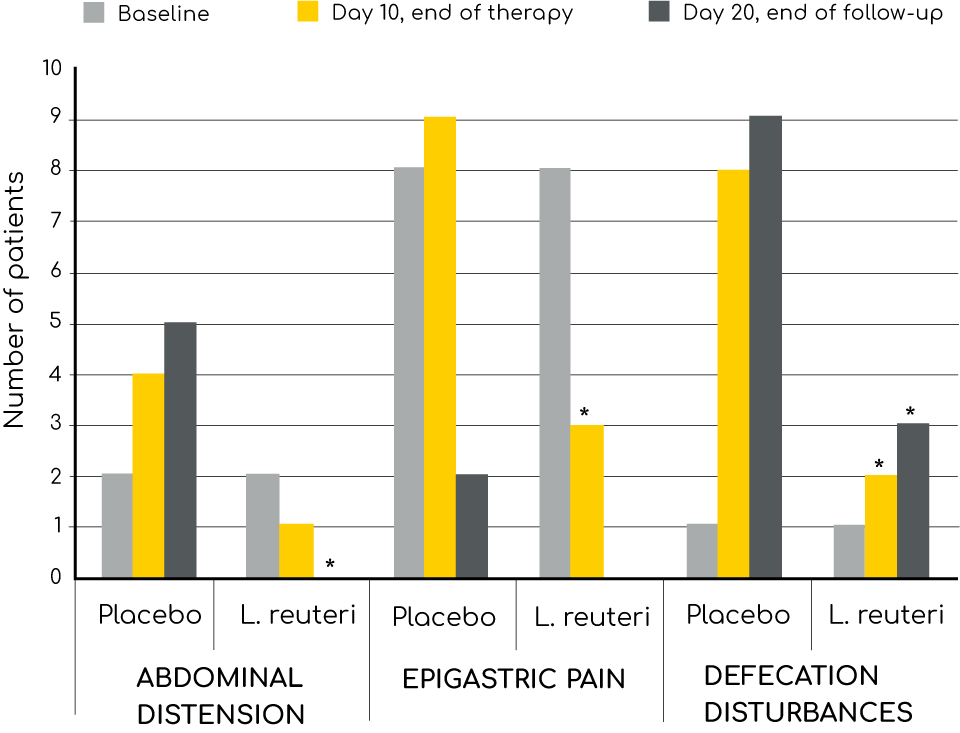Antibiotic Associated Diarrhoea (AAD)
Since antibiotics do not just kill the bad bacteria but also the good ones, the balance of the microflora is affected during the treatment. This may cause diarrhoea and other stomach problems, a condition called antibiotic associated diarrhoea (AAD). Other symptoms such as bloating, stomach pain and other functional abdominal symptoms may also occur.